医疗器械网络安全:符合美国/FDA标准
医疗器械网络安全:符合美国/FDA标准
为了在美国销售医疗器械,制造商必须符合食品和药物管理局(FDA)制定的既定医疗器械网络安全要求。这意味着制造商必须向FDA提交上市前申请,以确保符合市场准入要求。
FDA上市前要求
这些要求包括:
- 软件概述
描述重要的软件特性、功能、分析、输入、输出、硬件平台以及相关图表或流程。 - 开发和配置摘要
生命周期开发计划、配置管理和维护活动的摘要。 - 网络安全风险评估
包括风险分析、评估、控制以及FDA推荐的用于网络安全风险的SPDF使用的风险评估。 - 软件更新协议
软件更新、补丁管理、安全评估和网络安全应急响应计划的标准操作程序。 - 上市后监督计划
详细说明漏洞监控、漏洞识别规程和响应策略方法的计划。 - 软件物料清单(SBOM)
详细的SBOM,列出所有软件组件,包括版本号、许可证和供应商信息。 - 网络安全测试的证据
提供已进行网络安全测试的证据。
Applus+ Laboratories提供全面的网络安全测试服务,用于评估医疗器械是否符合美国/FDA医疗器械网络安全指南——质量体系注意事项和上市前提交内容。
网络安全测试和器械风险评估的文档级别
对于网络安全测试,以及标准要求的全部文档,定义了两个级别:基本文档级别和增强文档级别。
以下是一些不同级别应用示例:
- 一款非接触式红外测温仪,用于间断测量额头体温,不与其他设备连接。
- 理由:一般来说,在实施风险控制措施之前,器械软件功能的故障或潜在缺陷不会导致患者、器械使用者或使用环境中的其他人面临死亡或严重伤害的危险。
- 结果:基本文档级别。
- 用于治疗心动过缓的植入式心脏起搏器;该器械是一种植入式可编程双腔脉冲发生器,具有蓝牙功能。
- 理由:在实施风险控制措施之前,器械软件功能的故障或潜在缺陷(例如起搏失败或导致异位心搏错误感应的潜在缺陷)将导致危险情况,从而可能对患者造成死亡或严重伤害的风险。
- 结果:增强文档级别。
- 非患者匹配的髋关节假体,其中不包含固件或其他基于软件的控制手段。
- 理由:该器械不含软件。
- 结果:无文档级别
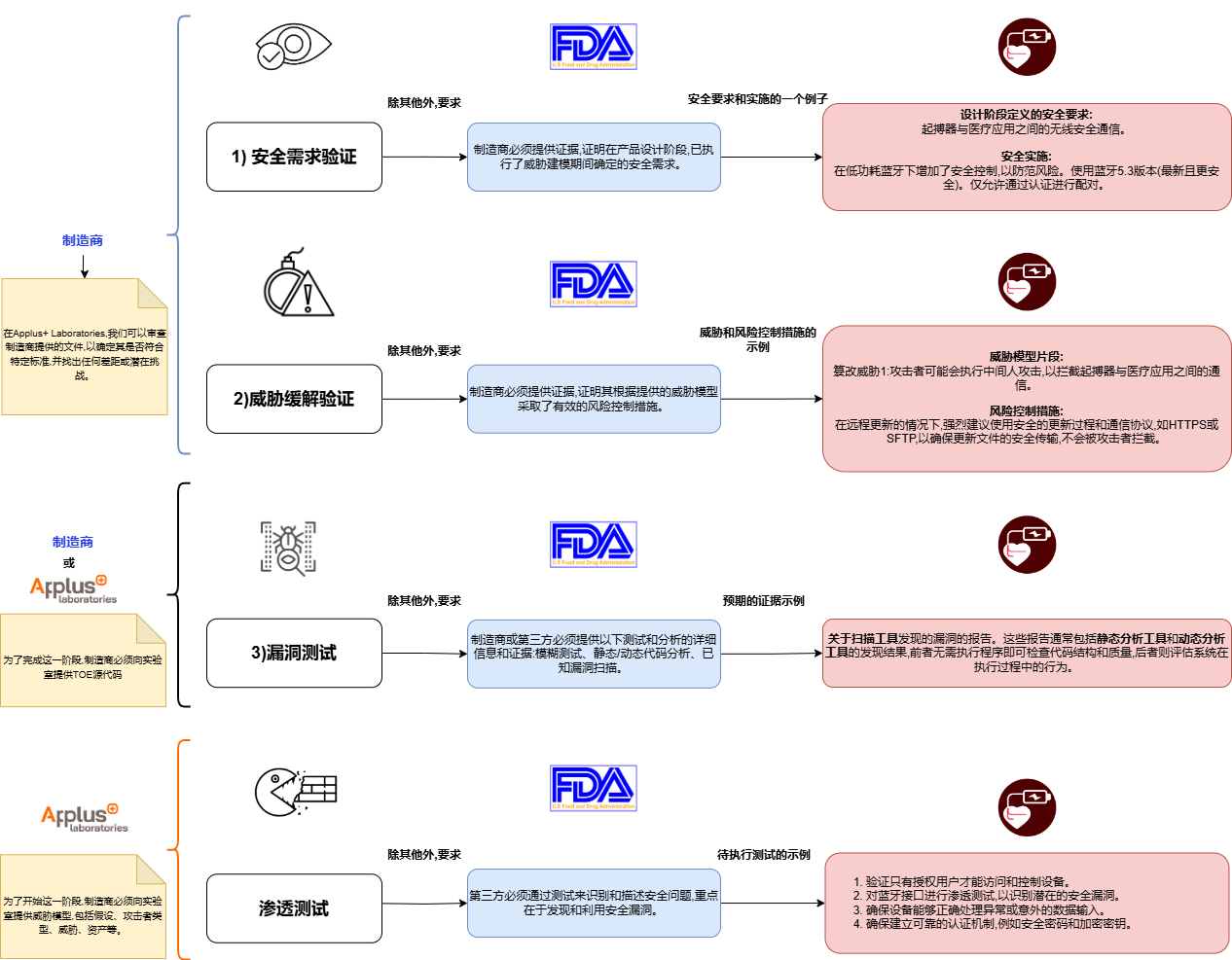
医疗器械文档程序
每个具有基本或更高文档级别的医疗器械都必须满足网络安全测试程序。满足网络安全测试标准所需的证据如下(以具有蓝牙功能的植入式心脏设备为例):
欢迎联系Applus+ Laboratories,测试并认证您的医疗器械的网络安全!